Proteins, peptides, enzymes, growth factors and more.
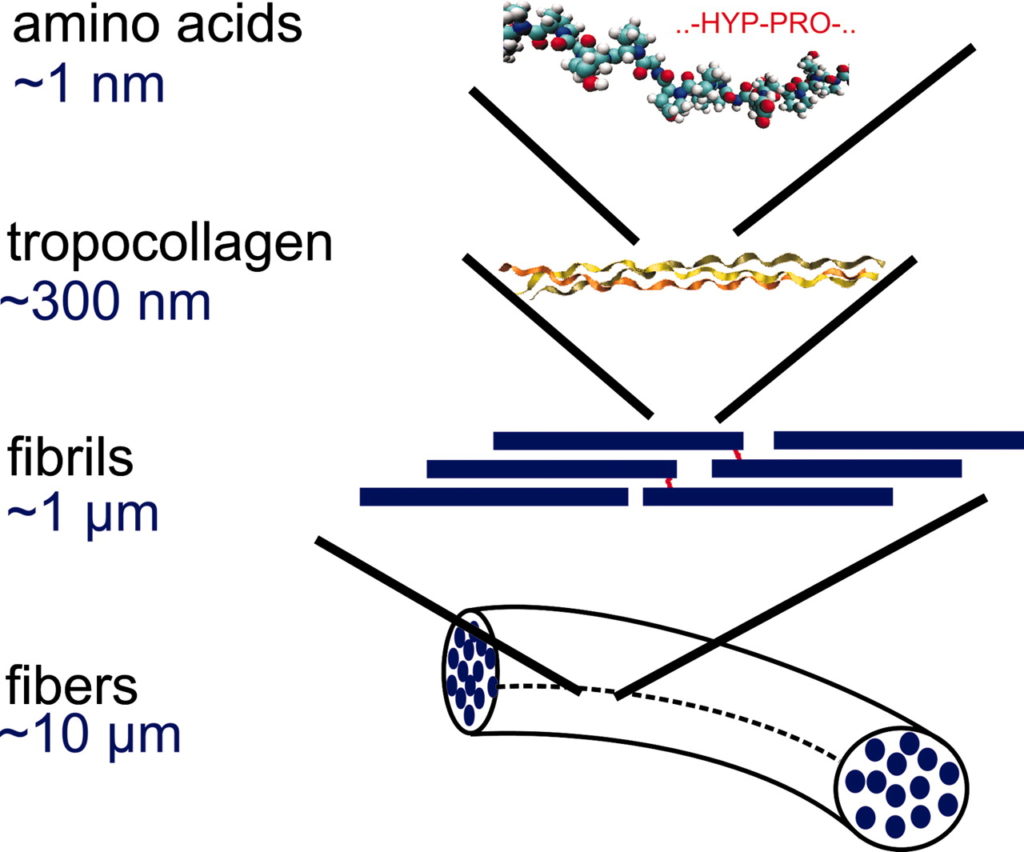
We can classify molecules by structure or by what they do. In the case of proteins, their structure can be very complex because they can do so many different things: they may be involved in maintaining a structure, like collagen or elastin, or they may be enzymes, accelerating chemical reactions so that life becomes possible. Or they may communicate information, like a growth factor telling a cell what to do.
General Structure: amino end – alpha carbon (+special radical- R group) – carboxyl end
Naturally occurring amino acids occur in two forms, which are stereoisomers of each other. The naturally occurring form in proteins is the L- (levo) form. In the L- form, the amino group is positioned to the left of the alpha carbon.
Amino acid characteristics are the result of their R groups. Amino acids are very often classified according to the properties of their R groups.
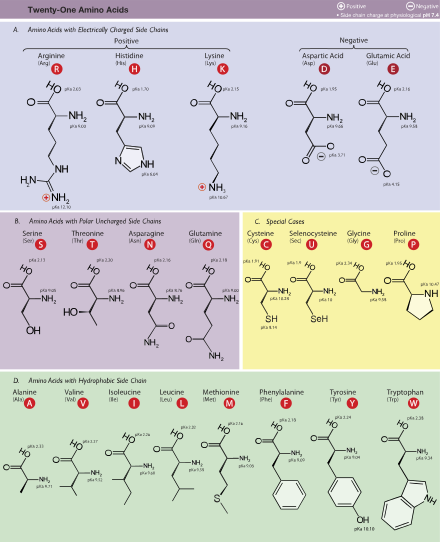
Figure. The amino acids. From Wikipedia.
1. Nonpolar – Glycine, Leucine: Amino acids with nonpolar side chains are hydrophobic
2. Polar, but uncharged – Serine, Cysteine, Asparagine: Cysteine is important due to its ability to form covalent bonds with other Cys. These amino acids are hydrophilic
3. Aromatic – Phenylalanine, Tyrosine, Triptophane: These amino acids are relatively nonpolar, so they are hydrophobic. However, Tyrosine can form hydrogen bonds through its -OH group and this group is also relatively reactive and can be covalently modified.
4. Negatively charged (acidic, good proton donors) – Glutamate, Aspartate: These amino acids are charged so they are hydrophilic
5. Positively charged (basic, bad proton donors) – Arginine, Histidine, Lysine: These amino acids are also hydrophilic. Important in electrostatic interactions between substances (like in DNA binding).
Amino Acids ionize in solution.
Ionic form at very low pH’s: +H3N-CH2-COOH (glycine, all dissociable H’s present)
At neutral pH: +H3N-CH2-COO- (the zwitterion form)
Ionic form at very high pH’s: H2N-CH2-COO- (Glycine has lost all its dissociable H)
At pH’s near 6, glycine can both donate and accept protons. The overall charge on a given amino acid and its relative ability to accept or donate protons will depend upon the solution’s pH. It is dissolved and the pKa’s of the ionizable groups.
Peptide bonds are formed by a condensation reaction and the elimination of a molecule of water. Long chains of amino acids form polypeptides (usually less than 100 AA’s) or proteins (if longer than 100 AA’s). Peptide bonds are rigid due to the partial double bond character.
Amino acids can form peptides. A dipeptide (two amino acids linked by a peptide bond), tripeptide (3), tetrapeptide (4), etc. When you don’t remember the name of numbers in Greek, use oligopeptide (oligo= few). Peptides are usually represented by a sequence of letters, one for each amino acid, and sometimes by three letters for each amino acid.

Figure: a peptide
Peptide bonds are formed by a condensation reaction and the elimination of a molecule of water. Long chains of amino acids form polypeptides (usually less than 100 amino acids) or proteins (if longer than 100 amino acids). Peptide bonds are rigid due to the partial double bond character.
Primary structure of a protein
The sequence of amino acids in a protein is referred to as its primary structure, and each amino acid within a protein molecule is often referred to as a residue. The primary structure reveals much about a protein. The number and location of cysteine residues indicate where disulfide bonds may form (an indicator of how proteins may fold).
Proteins often contain amino acids that have been modified. Lipids make up Lipoproteins, carbohydrates make up Glycoproteins, phosphate makes up Phosphoproteins, etc.
Why we need to be careful when dealing with proteins
Proteins can be denatured (i.e.- linearized) under any one of the following conditions:
1) Extremes of pH (disrupt ionic interactions and H-bonds)
2) detergents (SDS, Triton; disrupt the hyrophobic interactions)
2) Denaturing agents (that disrupt the hyrophobic interactions) like urea or guanidine
3)Alcohols like ethanol (also disrupt hydrophobic interactions)
The result of denaturation is a loss of protein function (like its enzymatic function). If the denaturing agent is then removed, the protein may reform
Protein Secondary Structure
There are a few common motifs that short stretches of amino acid sequences can spontaneously form:
1) Alpha helix – which is the most compact structure that a chain of amino acids can assume inter distance is 1.5 Angstroms and has 3.6 amino acids per turn. It is stabilized by intramolecular H-bonds every 4th amino acid and minimizes repulsion between R groups (which project toward the exterior of the helix); therefore, some amino acids (R too large, for example) are not commonly found in these structures
2) Beta conformation – is the most extended form an amino acid chain can assume; intra-amino acid distance is 3.5 Angstroms. Chains of amino acids in this conformation can be linked together by either intra- or interchain H-bonds to form a beta-pleated sheet.
3) Beta turn -often found connecting chains of amino acids in beta-sheet. Usually have a Pro because it is kinked or a Gly because its R group is small.
Protein Tertiary Structure
– is the result of interactions between the secondary structures and is the 3-D structure of the final protein.
– portions of the 3-D structure can be described in terms of protein domains. This refers to a functional region, like substrate binding site, oxygen binding site, catalytic region, ATP binding site, phosphorylation site.
The primary structure of a protein, i.e., the amino acid sequence, contains all the information needed to form a tridimensional structure.
Protein quaternary structure applies only to proteins that have more than one subunit. The association of subunits into a functional macromolecular unit is the quaternary structure.
Short peptides:
Short peptides (3-6 amino acids) are too simple to shape into the kind of spacial structure that can “fit” into anything of interest. They will be broken down and used. You may be able to “shorten” a protein and keep its function, but you have to demonstrate that this is possible. You can’t ask a short peptide to do the job of a complex protein.
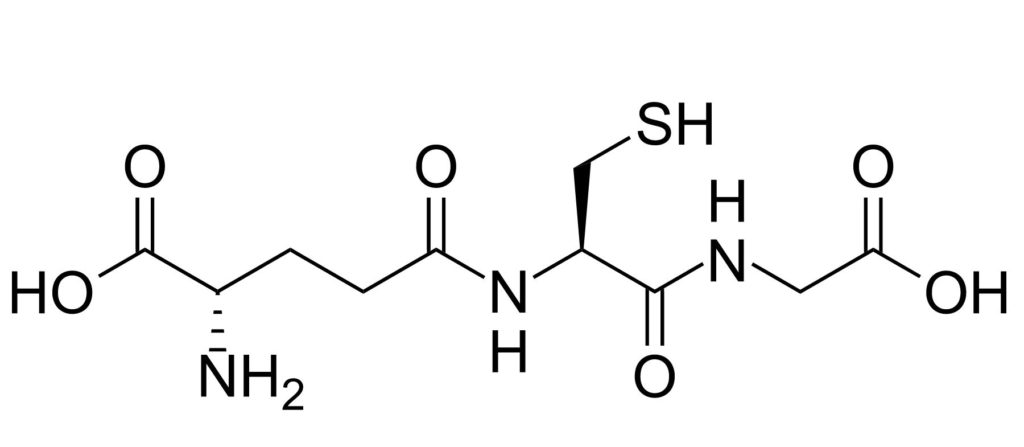
Figure: the peptide glutathione is short but powerful!
Glutathione (reduced) is a peptide that will help your skin face the oxidative stress caused by pollution and even our own metabolism.
Long peptides (polypeptides, short proteins).
Epidermal growth factor (EGF) is folded in such a way that will fit the EGF receptor and start a complex chain of chemical reaction in your skin that will result in more protein synthesis, more cell division, and smoother and nicer skin. One minute drop of this protein in a cream, lotion, or water-based serum, and your skin will be grateful to you.
