For fatigue, look at the mitochondria. Now there is proof.
Chronic fatigue is a debilitating symptom that affects many individuals, but what is the mechanism? A new study shows that a stress–induced protein called WASF3 localizes to mitochondria where it disrupts the assembly of the respiratory supercomplex, leading to decreased oxygen consumption and low exercise endurance. Alleviating endoplasmic reticulum (ER) stress decreases WASF3 and restores mitochondrial function, indicating that WASF3 can impair skeletal muscle bioenergetics. The good news? This protein is now the target for treating fatigue symptoms.
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) afflicts more than 2 million people in the USA. People with ME/CFS live with debilitating symptoms, including exhaustion, exercise intolerance, cognitive problems, and worsening symptoms after even mild exertion (known as post-exertional malaise). Many people first develop symptoms after a viral infection, but the lack of understanding of the mechanism limits both diagnosis and the development of treatments. The doctor has few tools to alleviate the illness.
A new study by NIH researchers (Hwang et al., 1923) shows that fatigue in several individuals correlated with a very slow recovery of cellular energy production after exertion and low oxygen used by mitochondria (the cell compartment that converts food into usable energy). The team identified the culprit, a protein called WASF3. The synthesis of this protein is boosted in response to cellular stress, and blocking WASF3 allows mitochondria to produce energy at normal levels. The extra WASF3 produced in the cells interfered with the formation of the structures that mitochondria use to produce energy. Most people with ME/CFS they studied had substantially higher levels of WASF3 than healthy subjects.
This dysfunctional increase in WASF3 seemed to be linked to the impairment of a cellular signaling pathway called the ER stress pathway. The ER stress response pathways are usually triggered by events such as oxidative stress or the buildup of unfolded protein in the ER. The researchers treated cells from the initial study participant with an experimental drug called salubrinal, known to reduce ER stress. After this treatment, WASF3 levels decreased in the cells, more mitochondrial energy complexes formed, and energy production improved.
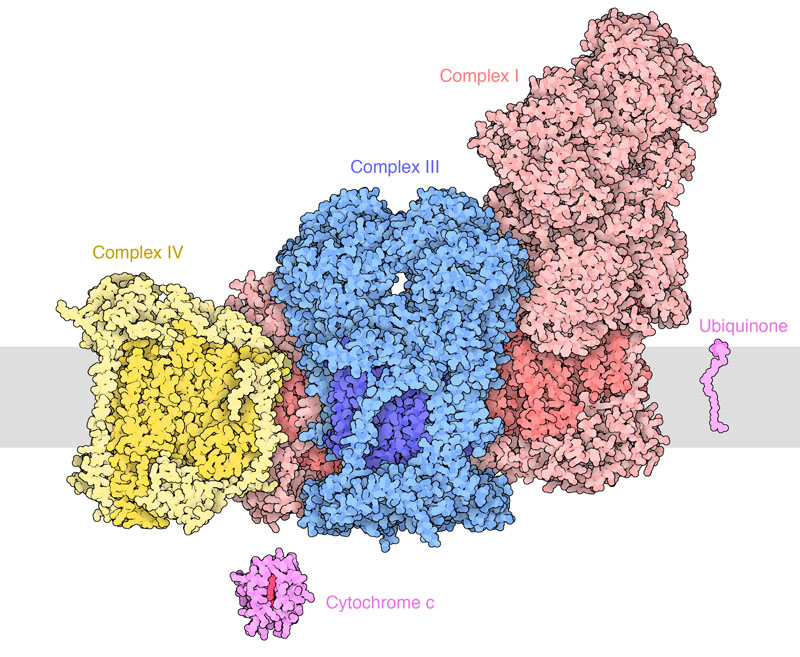
Figure: The respiratory supercomplex
Why is this discovery so important? Besides the many that suffer from poorly understood “chronic fatigue,” mitochondrial dysfunction has been found in some people with “long COVID” and other conditions, like rheumatic disease, that include fatigue. More research is needed to understand whether targeting ER stress may be a promising approach for these conditions. Even before remedies are easily available, there is now a way to easily identify if fatigue problems are related to an unusual accumulation of the stress protein WASF3.
What can you do for yourself? You can exercise! You can watch your diet! Talk therapy will help, too; those words eventually modify how your brain works. Studies have shown that supercomplex formation in skeletal muscle is promoted by exercise and is positively correlated with mitochondrial oxygen consumption, while metabolic dysfunction (diabetes, etc.) is associated with disruption of the supercomplexes. Too often, we forget that what we do with our body can change it for better and worse.
Is there anything that Skin Actives can do for you? Not for chronic fatigue, but yes, for taking care of the mitochondria in your skin. Look, for example, at our Revitalizing Nutrient Cream, full of goodies that will make your skin mitochondria happy.
References
