What’s special about Skin Actives Amino Booster?
What’s in it? Amino acids. That’s it.
Glutamine, Arginine HCl, Leucine, Serine, Cysteine HCl, Valine, Proline, Lysine HCl, Glycine, Asparagine, Glutamic Acid, Threonine, Alanine, Phenylalanine, Methionine, Aspartic Acid, Tyrosine, Tryptophan, Histidine HCl, Isoleucine.
General structure:
amino end – alpha carbon (+special radical- R group) – carboxyl end
Naturally occurring amino acids occur in two forms, which are stereoisomers of each other. The naturally occurring form in proteins is the L- (levo) form. In the L- form, the amino group is positioned to the left of the alpha carbon.
Amino acid characteristics are the result of their R groups. Amino acids are very often classified according to the properties of their R groups.
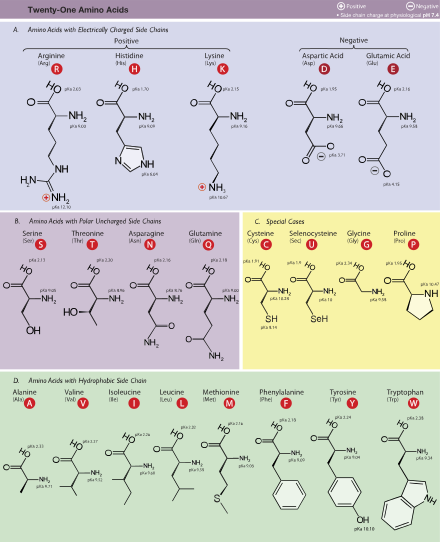
Figure. The amino acids. From Wikipedia.
1. Nonpolar – Glycine, Leucine: Amino acids with nonpolar side chains are hydrophobic
2. Polar, but uncharged – Serine, Cysteine, Asparagine: Cysteine is important due to its ability to form covalent bonds with other Cys. These amino acids are hydrophilic
3. Aromatic – Phenylalanine, Tyrosine, Triptophane: These amino acids are relatively nonpolar, so they are hydrophobic. However, Tyrosine can form hydrogen bonds through its -OH group and this group is also relatively reactive and can be covalently modified.
4. Negatively charged (acidic, good proton donors) – Glutamate, Aspartate: These amino acids are charged so they are hydrophilic
5. Positively charged (basic, bad proton donors) – Arginine, Histidine, Lysine: These amino acids are also hydrophilic. Important in electrostatic interactions between substances (like in DNA binding).
Amino Acids ionize in solution.
Ionic form at very low pH’s: +H3N-CH2-COOH (glycine, all dissociable H’s present)
At neutral pH: +H3N-CH2-COO- (the zwitterion form)
Ionic form at very high pH’s: H2N-CH2-COO- (Glycine has lost all its dissociable H)
At pH’s near 6, glycine can both donate and accept protons. The overall charge on a given amino acid and its relative ability to accept or donate protons will depend upon the solution’s pH. It is dissolved and the pKa’s of the ionizable groups.
What will your skin do with these amino acids?
Make proteins, whatever you need at the time and location.
What’s special about Skin Actives amino booster?
- The concentrations are chosen according to the needs of your skin cells (not your muscles or your liver)
- The quality: obtained by fermentation, these are the L-forms, the form that out bodies can use.
- They are suitable for vegans
What can the Skin Actives amino booster do to for you?
Remove amino acids as a limiting factor in your skin healthy metabolism.
Learn more about this concept in the posts below
Can skincare products become less effective over time? – Dr. Hannah Sivak, PhD | Skincare Expert
Why is the Revitalizing Nutrient Cream so complicated? – Dr. Hannah Sivak, PhD | Skincare Expert
DISCLAIMER: These claims have not been evaluated by the FDA and are not intended to diagnose, cure, treat or prevent any disease.
