What is acid layering? Is it good for your skin?
What is it? Acid layering consists in applying to your skin products with different acids, one after another.
Does it make sense to layer acids? No. If you are looking to acidify your skin, say, from the usual pH of 6 to 7 to something like 2 or 3, it doesn’t matter which acid you choose. Why? Because what matters is the concentration of protons being released by the acid, not the structure of the acid that releases the proton.
The pH of a weak acid solution is determined by the pK of the acid (which measures the tendency the acid has to give away its proton/s) and its concentration. You can use glycolic acid or malic, succinic or citric or lactic or whatever.
Play with acids? Don’t. Unless you know chemistry, you may very well end up in the emergency room with a chemical burn that will leave lasting scars. Even a chemist can end up with chemical burns if she is not careful. So don’t mess with acids.
My answer would be different if we were discussing specific acids. Maybe you are interested in a chemical not because it’s an acid but because of its structure and properties. For example, succinic acid seems to discourage the growth of acne bacteria. The salt of lactic acid, lactate, may help retain skin moisture.
Then, how did this “acid layering” story start? Like many other such stories, it probably started in an advertising team meeting with the question “how can we sell more products” in an already saturated market. The advertising team comes out with the idea which is then communicated to the formulator who has to will come up with something that is not dangerous but not necessarily useful either. Add to the mix some “influencers” and voila! a new myth is born. “Acid layering is good for your skin”. The testing will be done by (and paid for) by the customer, who after a couple of months will complain that now her skin is sensitive and has no idea why.
Of the acids used in skincare, there is one, salicylic acid (called beta by the industry just because they can) that has a proton to give, but that’s where the similarity stops. It’s not very water-soluble and has a different chemical structure from all those alpha hydroxy acids I mentioned above. Salicylic acid is used in exfoliation and to control acne. Although salicylic acid is an acid, it seems that its effect on desquamation may not hinge on the acidity, i.e. a similar effect can be obtained at neutral pH. Salicylic acid seems to work by disrupting cell to cell junctions rather than breaking intercellular keratin filaments. Salicylic acid also has the comedolytic effect, probably because it increases desquamation, keeping pores open, and making them unhospitable for acne bacteria.
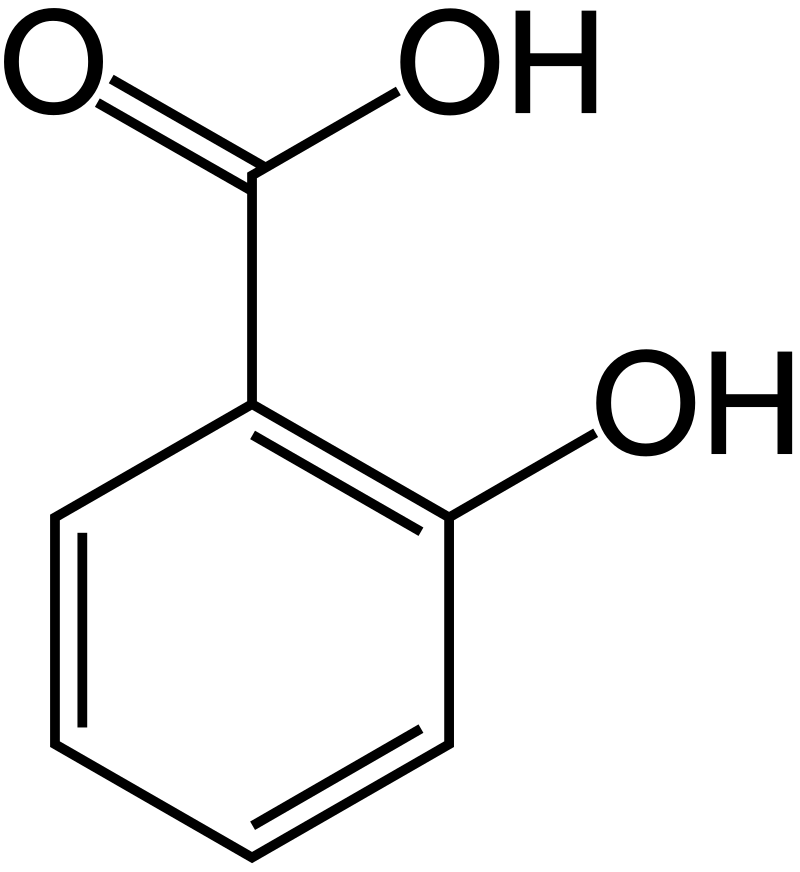
Figure. Chemical structure of salicylic acid. From https://commons.wikimedia.org/w/index.php?curid=1563860
Want to have a good mix of alpha and “beta” hydroxy acids? Go for Skin Actives alpha-beta exfoliator. It makes sense.
References
Merinville, E., Laloeuf, A., Moran, G., Jalby, O., & Rawlings, A. V. (2009). Exfoliation for sensitive skin with neutralized salicylic acid? International Journal of Cosmetic Science, 31(3), 243–244. doi:10.1111/j.1468-2494.2009.00501_2.x
Arif T. (2015) Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
DISCLAIMER: These claims have not been evaluated by the FDA and are not intended to diagnose, cure, treat or prevent any disease.
