Peptides in the cosmetic industry.
In chemistry, the structure and definition of “peptide” is straightforward: two or more amino acids joined by peptide bonds.
Amino acids are relatively small molecules: the smallest is glycine, with just two carbon atoms; one of the carbons is in the acid group (COO-) and the other has the amino group (NH4+) attached to it. Other amino acids have longer carbon chains; some have a couple (instead of just one) of amino groups or acid groups. There are many amino acids, but only 20 are common in proteins. Naturally occurring amino acids occur in two forms which are stereoisomers of each other. The naturally occurring form in proteins is the L- form. In this form the amino group is positioned to the left of the alpha carbon.
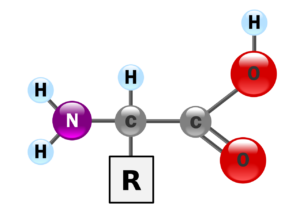
Figure: an amino acid
Amino acids can form peptides. A dipeptide (two amino acids linked by a peptide bond), tripeptide (3), tetrapeptide (4), etc. When you don’t remember the name of numbers in Greek, use oligopeptide (oligo= few). Peptides are usually represented by a sequence of letters, one for each amino acid, and sometimes by a sequence of three letters for each amino acid.

Figure: a peptide
Peptide bonds are formed by a condensation reaction and the elimination of a molecule of water. Long chains of amino acids form polypeptides (usually less than 100 amino acids) or proteins (if longer than 100 amino acids). Peptide bonds are rigid due to the partial double bond character.
The skin care industry uses synthetic peptides that mimic peptides occurring naturally and modifies them by attaching a fatty acid to one end. This became possible thanks to methods initially developed to facilitate the work of scientists, who needed synthetic peptides for their research. But things have changed in the last couple of years in a worrying way. The chemists working for companies that manufacture ingredients have been too innovative, drifting away from natural structures in their search for novelty. With novelty comes risk, but because these ingredients are sold to be used in “cosmetics” the research done by these companies to prove safety is just a formality. Usually there is no published research on these peptides, just a “white paper” that does not go through peer review.
Innovation: new for advertising’s sake
How does the industry get a “new peptide”?
1) Adding of a fatty acid to a peptide. This should be no problem because the bond will be broken by enzymes in the skin, resulting in the release of the peptide (or amino acids) and a free fatty acid.
2) Adding of a biotinoyl residue, i.e. Vitamin B7. Not useful but not harmful either, the bond is likely to be broken in the skin and the vitamin B7 can penetrate the cell membrane.
3) Creating a peptide that have no known function, like acetyl tetrapeptide-3. Here the “rationale” (not very rational, is to look at a structure used tissue engineering to make synthetic skin and choose a four amino acid sequence. The likelihood of this strategy working is nil but the risk is low.
4) Choose any sequence of any protein related to immunity (billions of options!) and hope that the chosen one will do something useful (as opposed to damage the skin, which is another possibility).
5) Now that peptide synthesis is getting less expensive, the industry is trying decapeptides (10 amino acids) or longer peptides. Same method, longer sequence, equally futile. What the sellers promise is to target important enzymes or receptors for signal proteins with these peptides. This is a fantasy designed for people who don’t know protein chemistry (obviously, most of the population!). If the people selling these peptides know that this is a fantasy, they are just lying to you. I sincerely hope it is “just” ignorance.
In theory, it is possible to “shorten” some proteins while keeping one or more of its functions intact. Achieving this is a complex task that involves building molecular models of receptor and ligand, docking models that predicts how the receptor and ligand interact, and finally build a model of a peptide that could replace the ligand. And all of this hard work is not enough, because a peptide is not stable enough to do the job in isolation, its spatial structure will change by the second as it is affected by water, ions and lipids that surround it. It is possible to do without the whole protein but you will need complex chemical “connectors”.
This is a major and expensive enterprise, and not one that will be used by companies looking for the fast buck..
6) The industry is even trying to change the meaning of the word “peptide: strange synthetic structures appear instead of amino acids linked by peptide bonds. This is dangerous territory: using amino acids from among the 20 nature used by living beings to build proteins, we can be sure that there are enzymes capable of breaking down the peptide bonds and releasing amino acids for our bodies to use or discard. Introducing new chemical structures into the bodies, topically or otherwise, should only be done after extensive in vitro and in vivo research. Consumers should not be used as Guinea pigs.
One example is Syn-Hycan, Tetradecyl Aminobutyroylvalylaminobutyric Urea Trifluoroacetate What a terrible idea!
Look at the structure of Syn-Hycan: (2S,5S,8S)-2,8-Bis(2-aminoethyl)-5-(1-methylethyl)-4,7,10-trioxo-3,6,9,11-tetraazapentacosanoic acid 2,2,2-trifluoroacetate
This is NOT a peptide. It is a derivative of gamma-aminobutyric acid that includes trifluoroacetic acid. Maybe its original use was in scientific experimentation and now the industry is testing it on your skin.
A common trick used by the skincare industry: “appropriate” a skin problem and claim that you have a cure for it, Tradenames are useful in this trick because they resemble the name of the skin problem you claim to fix.
Example: “progeline”. It sounds “scientific”, right?
It isn’t. There is no mention of it anywhere in the scientific literature.
But you can find the INCI for this ingredient: it is a solution of dextran (thickener), glycerin (humectant), a tripeptide L-Valine, N-(2,2,2-trifluoroacetyl)-L-valyl-L-tyrosyl- (a synthetic, fluorinated peptide), and water. The order of concentrations is more likely to be: water, glycerin, dextran, and tripeptide, with a preservative in there, somewhere, because otherwise the mix would have to be kept frozen to prevent bugs eating the glycerol within a couple of days.
Please note that our bodies are not used to see anything of the kind. We don’t have fluoride in our proteins! I have no idea how the people that designed the peptide came to the conclusion that it would inhibit cell senescence. Searching for the synthetic peptide in the scientific literature didn’t produce any results either. Another explanation is that fluorinated peptides are used in scientific resaerch so they may be available commercially.
For general information about what peptides can do for your skin, and what they can’t do (much more frequent) please see my posts https://hannahsivak.com/blog/understanding-peptides-diy-recipes-with-peptides-and-how-to-use-them/, https://hannahsivak.com/blog/peptides-in-the-cosmetic-industry/.
There is something very disquieting about the use of these fluorinated peptides in skincare. They are used in research to modify proteins, but they have no backing from clinical studies. For all intents ad purposes, the company selling you products containing synthetic peptides is using you as a Guinea pig.
What will they think of next? Caveat emptor, let the buyer beware.
If in doubt, write to us at Skin Actives and we will (try) to decipher suspicious ingredient lists.
Wang, A. S., & Dreesen, O. (2018). Biomarkers of Cellular Senescence and Skin Aging. Frontiers in Genetics, 9. doi:10.3389/fgene.2018.00247
Buer, B.C., B.L. Levin and E.N.G. Marsh (2012). “Influence of Fluorination on the Thermodynamics of Protein Folding”. J. Am. Chem. Soc, 134, 13027 – 13034
Suzuki, Y., B.C. Buer, H.M. Al Hashimi and E.N.G. Marsh (2011). “Using Fluorine NMR to Probe Changes in Structure and Dynamics of Membrane-Active Peptides Interacting with the Lipid Bilayer”. Biochemistry, 50, 5979–5987
